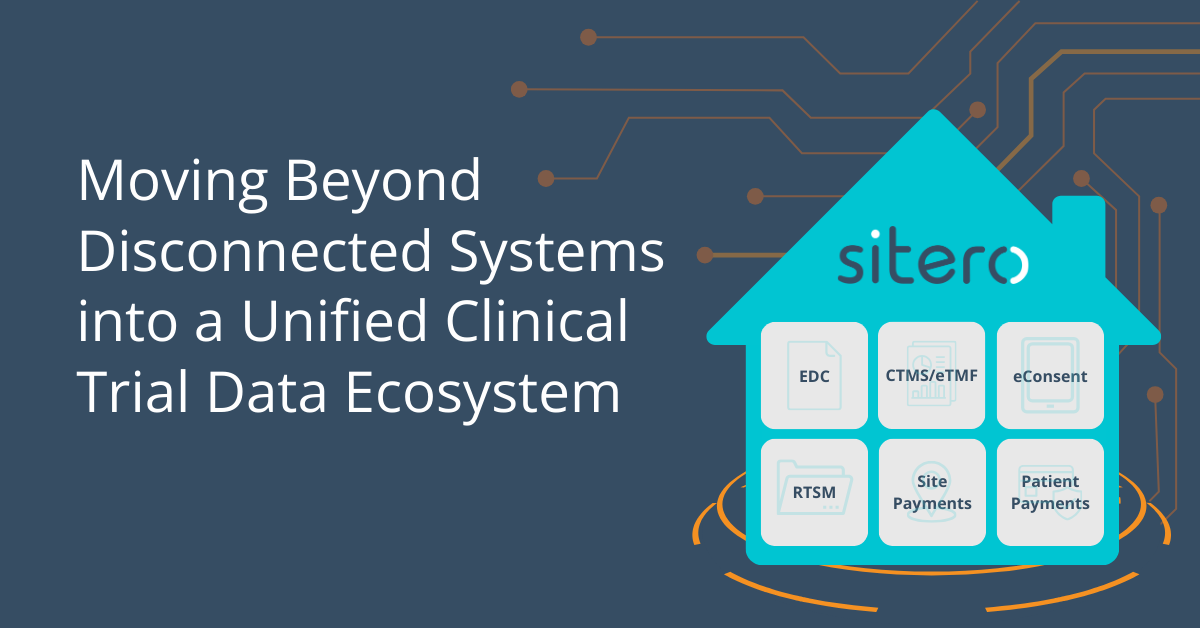
The future of clinical trials is a unified, interoperable ecosystem. Sitero’s Mentor platform is leading this transformation, ensuring that clinical trials are more efficient, compliant, and streamlined than ever before. Learn more in our latest blog:
Today’s trials require data entry from anyone, anywhere including patients, sites, home nurses, and remote investigators. Learn why EDC systems must evolve to support decentralized trails in our latest blog:
Learn how a fully configurable EDC with integrated ePRO and seamless workflows can eliminate inefficiencies and accelerate clinical trials in our latest blog:
Explore the benefits of outsourcing local pharmacovigilance (PV) and key strategies for ensuring compliance while optimizing costs and efficiency in our latest blog:
Clinical research starts with the site. But growing trial complexity and administrative burdens put significant strain on site teams. How can the industry better support them? Learn more in our latest blog:
Stay informed on the latest US policy for Dual Use Research of Concern (DURC) and Pathogens with Enhanced Pandemic Potential (PEPP), effective May 6th, 2025 in our blog:
Discover the benefits of the sIRB ruling for multi-site clinical trials to streamline ethical standards across sites in our blog:
Discover the diverse world of research compliance review boards and the roles they play, including IRBs, SCROs, DSMBs, IACUCs, and IBCs, in our latest blog:
Discover the true function of Electronic Trial Master File (eTMF) solutions and explore the benefits of integrating them with CTMS. Learn more in our blog:
In this post, Sitero summarizes the most recent amendments to the NIH Guidelines, covering key changes for GDMOs. Learn more in the blog:
Blogrobgjryan2025-04-16T16:10:52+00:00