NIH Announces Updates to the Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules
Author: Ryan Bartlett, MS, CPBCA, RBP, CBSP (ABSA), Biosafety Officer
On April 5, 2024, the National Institutes of Health (NIH) published the most recent changes to the Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines) in the Federal Register. Effective September 30, 2024, these changes are significant as they introduce specifications for conducting research involving gene drive modified organisms (GDMOs).
These updates come after a public consultation period, during which the NIH actively sought and considered input on proposed changes detailed in an initial Federal Register notice released on August 10, 2023. In response to the valuable feedback, the NIH clarified minimum containment requirements, offered guidance for conducting risk assessments, and outlined further institutional responsibilities concerning Institutional Biosafety Committees (IBCs) and Biological Safety Officers (BSOs).
The most recent amendments to the NIH Guidelines are meant to accomplish three main points about GDMOs.
- Clarify minimum containment requirements for research involving GDMOs.
- Provide considerations for risk assessments.
- Define additional institutional responsibilities for IBCs and BSOs.
In this post, Sitero summarizes the key changes for GDMOs and the other revisions to the NIH Guidelines.
April 2024 Amendments to NIH Guidelines
Gene Drive Definition
The Guidelines now incorporate a definition of Gene Drive. This term is defined as follows: “A technology whereby a particular heritable element biases inheritance in its favor, resulting in the heritable element becoming more prevalent than predicted by Mendelian laws of inheritance in a population over successive generations” (Section I-E-7).
Considerations for Risk Assessment
Section II-A-3 now includes guidance emphasizing the necessity for further risk assessments concerning GDMOs at your institution. The Guidelines highlight risks to be considered, such as:
- The specific types of manipulations, including the source of the genetic material or whether the gene drive can spread or persist in the local populations.
- Options for risk mitigation for specific types of risks in experiments or when dealing with uncertain risks.
- Considerations for implementing more stringent containment measures until biosafety data exists to support lowering containment levels.
Gene Drive Modified Organism Updates
Sections III-C-1 and III-F-1 have been updated to include language about introducing “stable genetic modifications into the genome.”
Sections III-D-4 and III-D-5 have been updated to include clarifying language about GDMOs that has been added throughout The Guidelines.
These sections also state that GDMO research with whole animals and whole plants shall be conducted at a minimum of Biosafety Level 2 per Section III-D-8. The Federal Register publication does note that your institution may petition NIH OSP for a lower biosafety level designation for work with GDMOs. OSP will review the requests on a case-by-case basis.
Section III-D-8 is a new section added to the Guidelines.
“Experiments involving gene drive modified organisms generated by recombinant or synthetic nucleic acid molecules shall be conducted at a minimum of Biosafety Level (BL) 2, BL2-N (Animals) or BL2-P (plant) containment.”
Additional sections throughout the Guidelines have been updated to include GDMO language (i.e., Sections III-E-3, III-F-1).
Section IV
Section IV has been updated to expand upon the roles and responsibilities of the institution. If your institution conducts research with GDMOs, you shall:
- Have a Biosafety Officer (who is also a member of the IBC) if the institution conducts research with GDMOs (Section IV-B-1-c).
- Ensure the IBC has adequate expertise (e.g., species specific containment, environmental risk assessment) using ad hoc consultants, if necessary when conducting GDMO research (Section IV-B-2-a-(1)).
- Assess the impact on the ecosystem if a GDMO were to be released (Section IV-B-2-a-(1)).
Section V-N
Your institution must also evaluate the potential for a GDMO to impact the ecosystem. The Guidelines outline this in Section V-N:
Determination of whether a gene drive modified organism has a potential for serious detrimental impact on managed (agricultural, forest, grassland) or natural ecosystems should be made by the Principal Investigator and the Institutional Biosafety Committee, in consultation with scientists knowledgeable of gene drive technology, and of the environment, and ecosystems in the geographic area of the research.
Section III-D-3
Section III-D-3 has been updated to replace the term “helper virus” with “helper system” throughout the section. This better captures the processes by which viral vectors can be produced in vitro.
Reclassification of Viral Risk Groups
The NIH has reclassified the Saint Louis encephalitis virus (SLEV) and West Nile virus (WNV) as risk group 2 (RG2) organisms, which aligns with the CDC’s classification of these viruses.
Conclusions
Following the feedback, the NIH developed a supplementary reference document titled “Biosafety Considerations for Contained Research Involving Gene Drive Modified Organisms.” This resource aims to provide additional guidance and resources for institutions, investigators, and the biosafety community.
These updates reflect the NIH’s commitment to fostering responsible research practices and ensuring the safe and ethical conduct of studies involving gene drive modified organisms within contained research settings. Interested parties can visit the NIH Office of Science Policy website for further information and access to the complete set of revised NIH Guidelines.
For any inquiries, individuals can contact Caroline Young, Acting Director of the Division of Biosafety, Biosecurity, and Emerging Biotechnology Policy, Office of Science Policy, at (301) 496-9838 or via email at SciencePolicy@od.nih.gov.
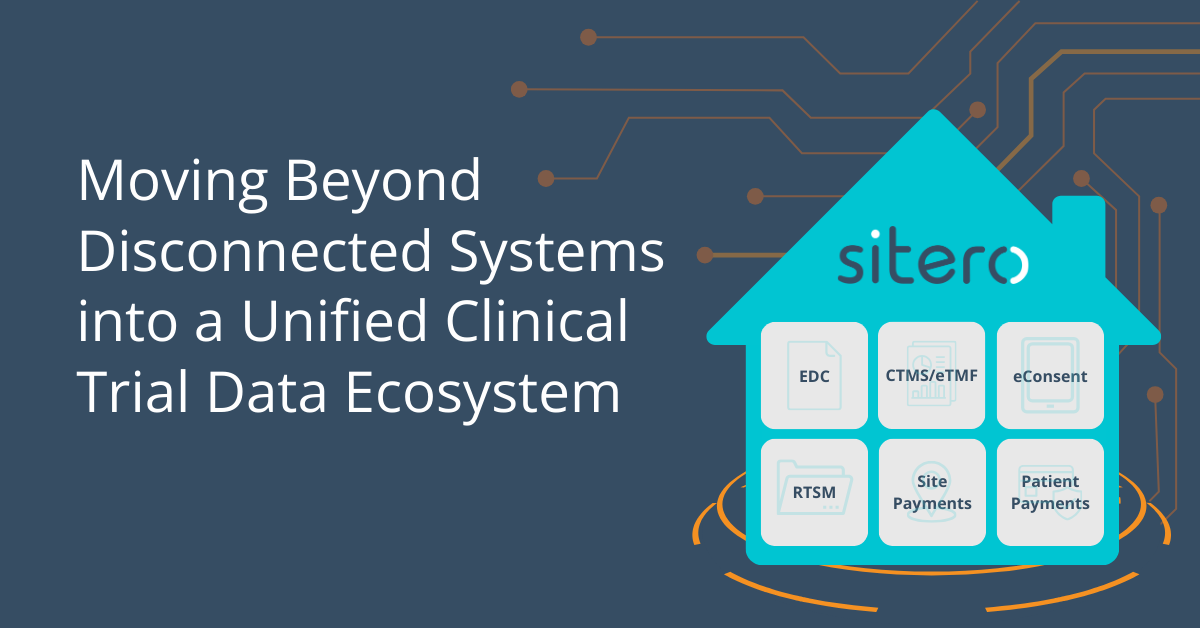
The future of clinical trials is a unified, interoperable ecosystem. Sitero’s Mentor platform is leading this transformation, ensuring that clinical trials are more efficient, compliant, and streamlined than ever before. Learn more in our latest blog:
Today’s trials require data entry from anyone, anywhere including patients, sites, home nurses, and remote investigators. Learn why EDC systems must evolve to support decentralized trails in our latest blog: