How Biosafety Adds Value to Research and Science
Authors: Sitero Biosafety Advisory Board
Biosafety is critical for scientific research and healthcare and is far more than just a set of regulations and guidelines. The biosafety discipline aims to keep researchers, the public, and the environment safe. But beyond just compliance and safety, biosafety adds tremendous value to scientific endeavors and research laboratories.
It is important first to understand what biosafety is and its role in research. Biosafety has many critical impacts on the quality and credibility of research and science. It is also essential to look at the future of biosafety and contemplate the evolving role as scientific advances continue to reshape the boundaries of knowledge and experimentation. By understanding the value that biosafety adds to research and science, we can navigate the path toward a safer and more robust scientific research environment.
What is Biosafety?
Biosafety is the study of biological hazards using evidence-based risk assessment and mitigation measures to prevent accidental exposure to a biological hazard or release to the environment. Based on these scientific inquiries, biosafety professionals develop practices and procedures that, when combined with administrative controls, engineering controls, and personal protective equipment (PPE), reduce the risk of exposure to biological hazards.
The risk assessment is primarily characterized by the biological material’s properties, such as:
- virulence/toxicity
- route of exposure
- transmissibility
- severity of disease
- genetic alterations
- indigenous vs. exotic, etc.
A robust risk assessment conducted by biosafety professionals takes all of these factors into consideration and recommends mitigation measures to protect the individual, community, and the environment. Additionally, factors such as the work performed, and the equipment used can also impact the overall risk. For example, aerosol-generating procedures, working with animals, and sharp devices increase the overall risk of a project.
Why is Biosafety Important?
Biosafety ensures that vital work with biological hazards can be done safely with as little risk as possible. A comprehensive biosafety program allows for robust risk identification and mitigation measures to conduct science and research activities safely.
Biosafety offers proven strategies to minimize the negative impacts of working with biological materials, including emerging and re-emerging infectious agents and existing, new, and evolving technology. As a result, biosafety protects:
- Workers against biological exposures and laboratory-acquired illnesses.
- Public health/communities against accidental and intentional exposures to biological materials.
- The environment against accidental and intentional releases of biological materials.
A well-developed biosafety program also promotes a good safety culture and helps minimize incidents involving biological materials, potentially resulting in the loss of institutional assets and reputational damage. Additionally, biosafety improves the quality of science and research programs. It minimizes cross-contamination risks to provide reliable and valid experimental results and helps to support laboratory accreditations and certification efforts.
The Future Role of Biosafety in Science
There has been increased regulatory oversight of biological research, both in the US and internationally, and it is likely that this trend will either continue or accelerate. Over the past 20 years, the US has seen the creation of the current Select Agent regulations, which set up an external regulatory permitting system for the possession and use of certain high-risk pathogens and toxins.
Changes in the import permit system over the past decade have also created a system for external review of laboratories importing biological agents and materials. Other countries, such as Canada, have created a licensing and inspection program for laboratories handling organisms that can cause disease and internationally the release of ISO35001, “Biorisk Management For Laboratories And Other Related Organisations,” has created a standard for the creation and maintenance of biosafety programs.
Recent proposed changes include the February 2023 recommendations (Proposed Biosecurity Oversight Framework for the Future of Science) from the National Science Advisory Board for Biosecurity (NSABB) that would extend Federal review of any work that could be “reasonably anticipated” to result in increased pathogenicity of an agent.
Additionally, the National Institutes of Health (NIH) Office of Science Policy (OSP) requested input into changes in the NIH Guidelines overseeing recombinant or synthetic nucleic acid research, specifically regulating the use of technology known as “gene drive” (Proposed Changes to the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules).
Looking towards the future, biosafety programs will need to continue to evolve as well, serving as partners and advisors to the research community, keeping them abreast of proposed changes in requirements, seeking their input, and advising them on how to balance research that furthers science and protects the public from the risks involved in an accidental release of a biological agent. Researchers alone cannot focus on their science and the regulatory environment; partnership will be essential.
Conclusions
In summary, biosafety is not just a set of regulations but an essential part of scientific integrity, public safety, and environmental well-being. It adds value by ensuring researchers can confidently push the boundaries of knowledge. As biosafety’s role continues to evolve in response to regulatory changes, collaboration between scientists, regulatory authorities, and biosafety professionals is key.
At Sitero we’re committed to enhancing your research endeavors and ensuring the safety of your research and the public. Join us in the journey to safer, more robust scientific exploration. Contact us today to see how we can help you navigate the path to a safer and more successful research environment through our biosafety services. Your safety is our priority.
About the Authors
Sarah Ziegler, Ph.D., RBP, CBSP (ABSA)
 Vice President of IRB, IBC and Biosafety
Vice President of IRB, IBC and Biosafety
Sarah Ziegler, Ph.D., is responsible for leading the Biosafety and IBC services at Sitero. Dr. Ziegler is an ABSA certified biosafety professional and is passionate about ensuring the safety of workers and the community during biomedical research. She has previously served as an IBC chair, Biosafety Officer and Responsible Official for multiple research institutions. She has also consulted on projects as an SME for Select Agents, Biorisk Management, facility design and Laboratory Operations. Dr. Ziegler was previously the Deputy Director of the National Bio and Agro-Defense Facility (NBAF) with the USDA where she supervised laboratory operations and Biorisk management. Dr. Ziegler received her Ph.D. from the University of Texas Medical Branch in Biomedical Science and was a Post-doctoral Fellow in the National Biosafety and Biocontainment Training Program (NBBTP).
Sabena Blakeney, Ph.D., RBP, CBSP (ABSA)
 Biosafety Advisory Board Member
Biosafety Advisory Board Member
Sabena Blakeney, Ph.D., serves as a subject matter expert on biological safety as a Sitero consultant. Her experience includes developing biosafety and select agent programs and associated documents, executing laboratory audits, incident investigations, training activities, and assisting with IBC approvals on human gene transfer studies at clinical trial sites. She has also performed biosafety reviews of laboratory drawings and advised on workflows and biorisk concerns. Additionally, Dr. Blakeney has over 10 years of laboratory research experience, including four years at Plum Island Animal Disease Center on research involving foreign animal disease control and eradication measures. She completed a post-doctoral fellowship with the National Biosafety and Biocontainment Training Program and is a Certified Biological Safety Professional (CBSP) and Registered Biosafety Professional (RBP) through ABSA International.
Paul Meechan, Ph.D., RBP, CBSP (ABSA)
 Biosafety Advisory Board Member
Biosafety Advisory Board Member
Paul Meechan. Ph.D., has expertise in biosafety risk assessments, regulatory requirements, laboratory operations, design reviews of new facilities, and biosafety training management. Before retiring from the CDC, he was the Associate Director for Laboratory Science in the Office of Laboratory Science and Safety. He also served as the CDC editor for the 6th edition of the NIH/CDC manual Biosafety in Microbiological and Biomedical Laboratories. He previously worked as the Director of the CDC’s Environment, Safety, and Health Compliance Office and the Corporate Biosafety Officer at Merck and Co. Dr. Meechan holds RBP and CBSP certifications from ABSA International and has served as President and Secretary of the ABSA Council.
Antony Schwartz, Ph.D., CBSP (ABSA)
Biosafety Advisory Board Member

Antony Schwartz, Ph.D., is an ABSA International Certified Biological Safety Professional and a microbiologist with extensive experience in biological risk assessment, clinical biosafety, and laboratory biosecurity solutions. Dr. Schwartz has held roles as the Institutional Biosafety Officer and Select Agent Program Responsible Official with the U.S. federal government and academic institutions. During the COVID-19 pandemic, he developed biosafety policies and procedures for clinical trials and research laboratories working with the SARS-CoV-2 virus and patient samples, as well as recommendations for personnel protective equipment (PPE) for healthcare staff. He led a team that implemented a large-scale N95 decontamination and reuse process for a healthcare facility. Dr. Schwartz is an active member of ABSA International currently serving as a Councilor.
References
- Schwartz, Antony, Andrea Vogel, and Mary Brock. “Biosafety Needs to Redefine Itself as a Science.” Issues in Science and Technology 39, no. 3 (Spring 2023): 72–73.
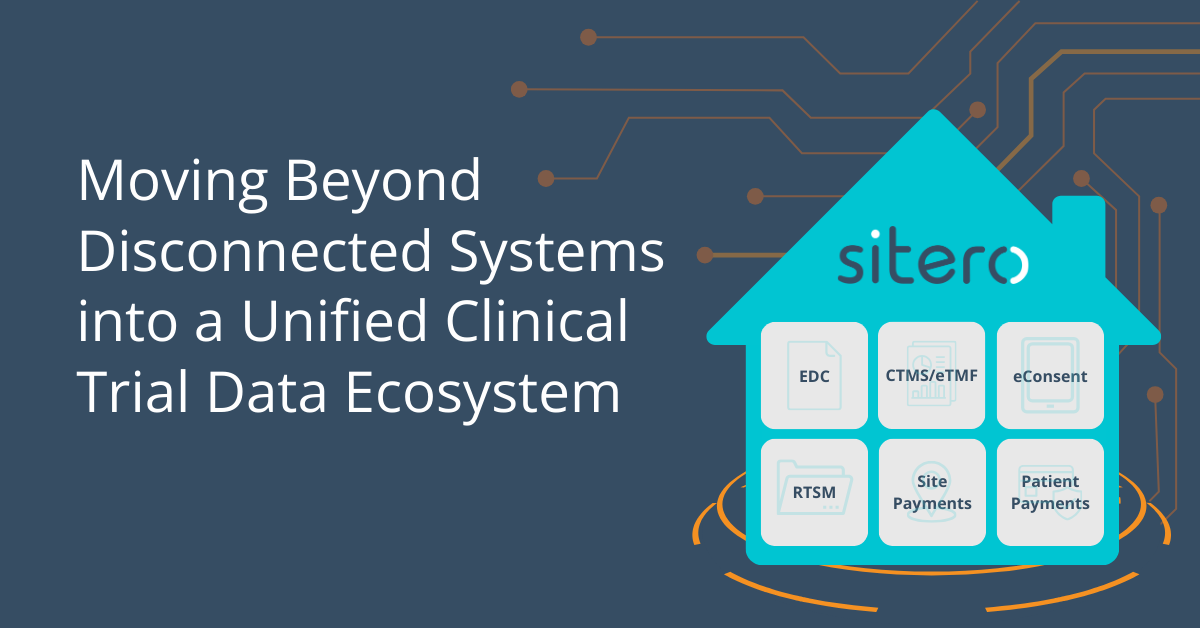
The future of clinical trials is a unified, interoperable ecosystem. Sitero’s Mentor platform is leading this transformation, ensuring that clinical trials are more efficient, compliant, and streamlined than ever before. Learn more in our latest blog:
Today’s trials require data entry from anyone, anywhere including patients, sites, home nurses, and remote investigators. Learn why EDC systems must evolve to support decentralized trails in our latest blog: