LATEST UPDATES
Major Release Coming July 2025!
Mentor CTMS is continuously evolving to meet real-world site and sponsor needs. Check back for platform updates and new CTMS + eTMF functionality.

Why Mentor CTMS?
Mentor CTMS Key Metrics
40+ CLIENTS | 2,000+ STUDIES | 800+ INTEGRATIONS | 50+ COUNTRIES SERVED
Mentor CTMS Features
Mentor CTMS is packed with practical, proven features—like embedded eTMF, global cloud hosting, and configuration wizards
to help you manage trials smarter and faster.
Two Decades of Well-Proven Functionality
Cloud Hosting Delivered Globally
Search Criteria Fields Maintain Study Context
Configuration Wizards Maximize Reuse
Embedded eTMF Module Within CTMS
Key Differentiators
Designed to centralize trial oversight and streamline workflows, Mentor CTMS offers real-time insights, automated processes, and faster site engagement from startup to closeout.
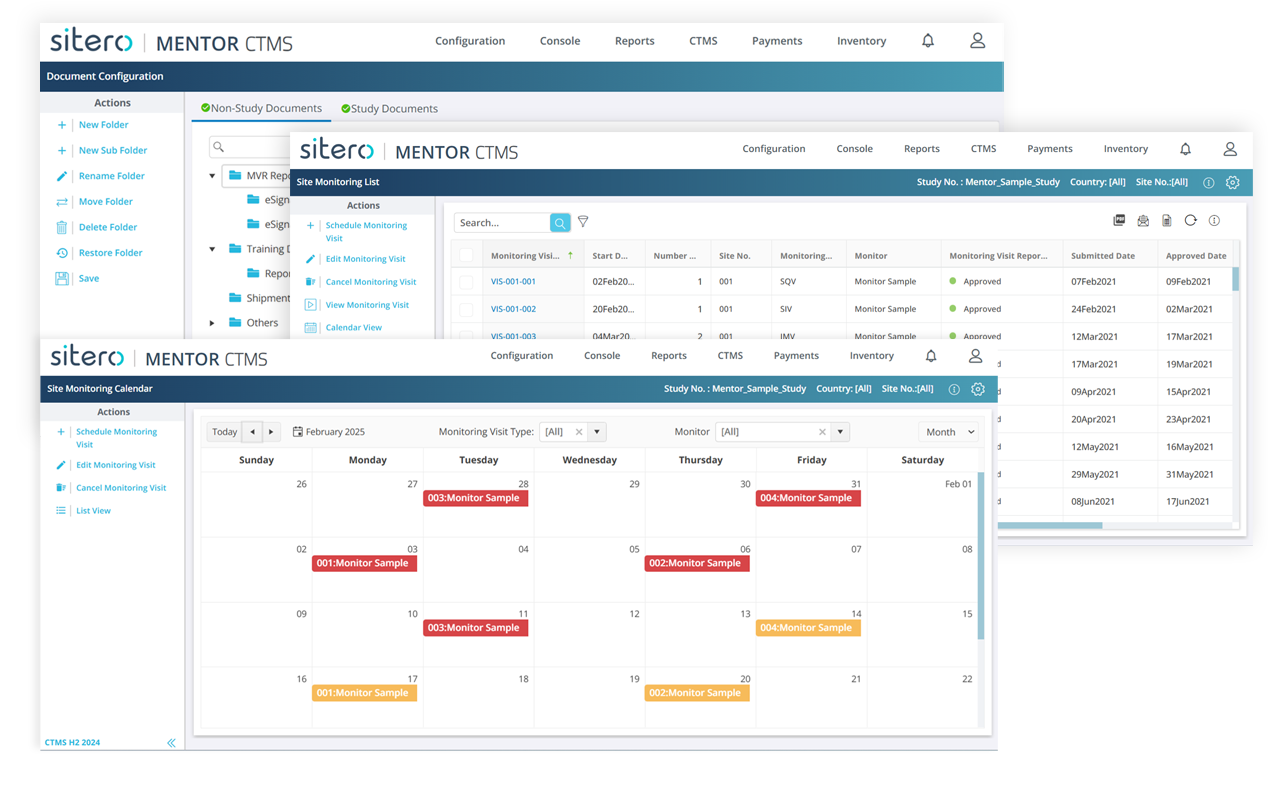
CTMS Accelerated Implementation
Mentor CTSM offers quick-start deployment options and hands-on support, your study gets up and running — without the heavy lift of traditional systems.
Testimonial
“We chose Sitero Mentor as our partner for CTMS because their product provides the functionality and flexibility to allow us to efficiently manage our clinical operations now and in the future, where we expect to manage more studies internally. With Mentor CTMS, we were able to cost-effectively implement a solution that supports core functionality today and allows us to easily expand with additional modules as our needs grow.”
Director of Information Technology
INDUSTRIAL BIOTECHNOLOGY COMPANY
CTMS Resources
Explore our blog for helpful tips and trends in clinical trial oversight. More resources and product updates coming soon.
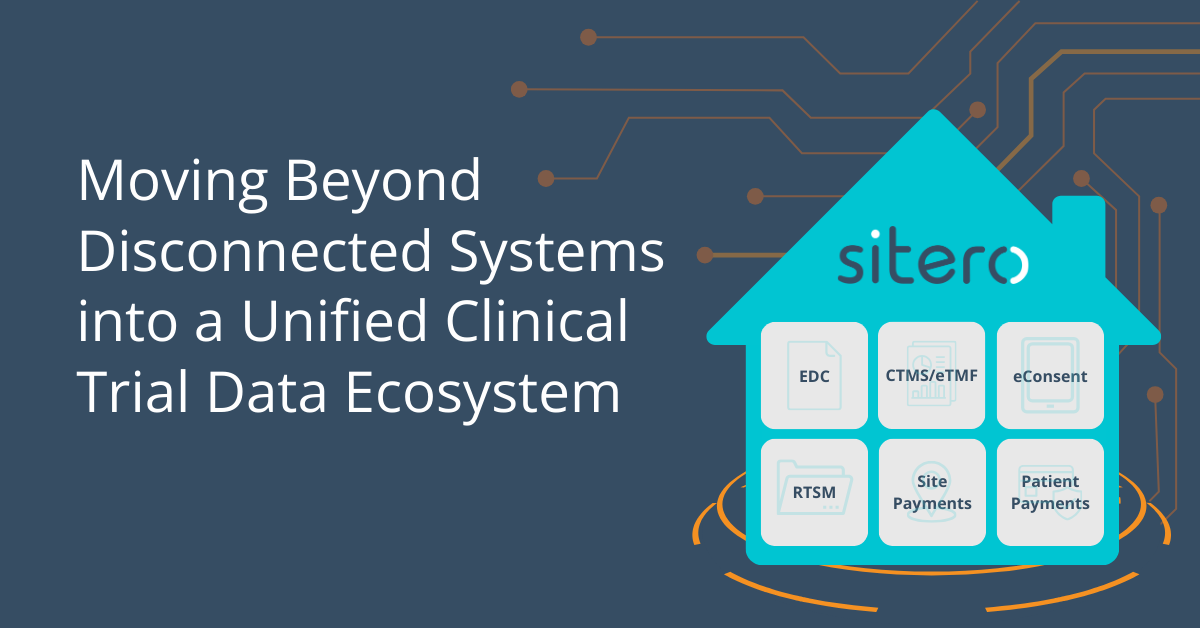
The future of clinical trials is a unified, interoperable ecosystem. Sitero’s Mentor platform is leading this transformation, ensuring that clinical trials are more efficient, compliant, and streamlined than ever before. Learn more in our latest blog:
Discover the true function of Electronic Trial Master File (eTMF) solutions and explore the benefits of integrating them with CTMS. Learn more in our blog: