More than a Signature.
Consent is one of the first and most important
patient touch points.
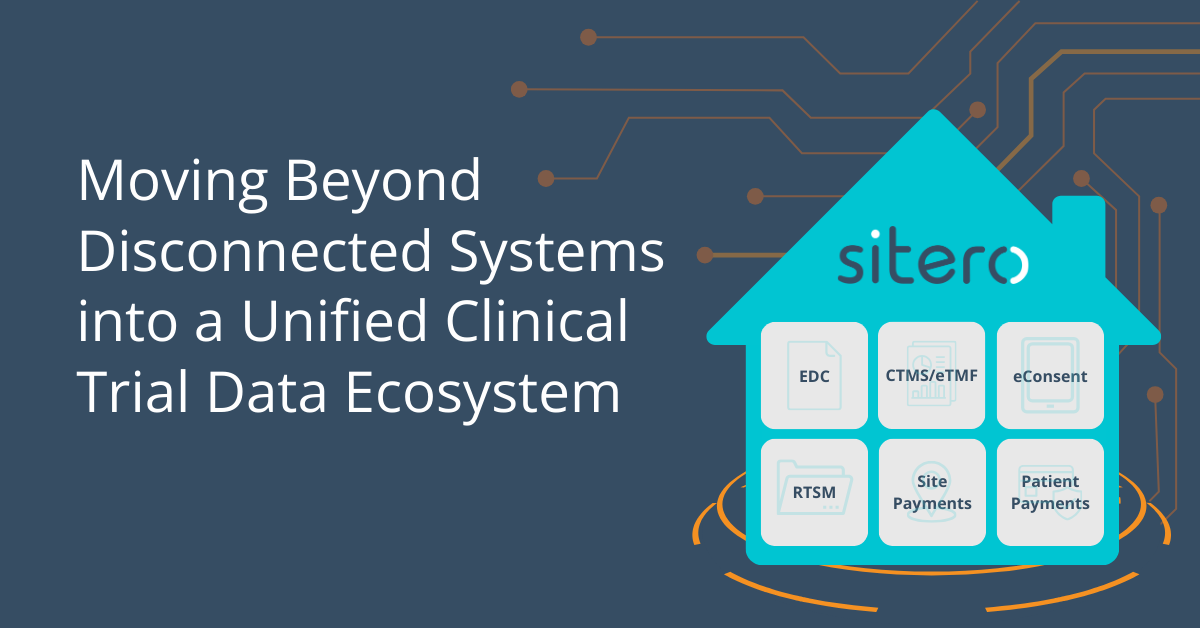
Sitero’s unique eConsent offering is adaptable and made for modern clinical trials. It is designed to meet the real-world needs of sponsors, CROs, sites, IRBs, and most importantly, clinical trial participants.
NATIVELY INTEGRATES WITH:
LATEST UPDATES
Enhanced Feature Set Launched April 2025!
Check back regularly for the latest improvements and platform news.

Why Mentor eConsent?
Mentor eConsent Key Metrics
30+ CLIENTS | 100+ STUDIES | 1,000+ USERS | 50 COUNTRIES SERVED
Mentor eConsent Features
Built for global flexibility and regulatory confidence. Designed to integrate seamlessly with RTSM and EDC. Multimedia and multilingual options ensure participants make informed decisions.
Out of the Box Template Libraries
Fully Enabled for Remote Consent Used Globally
Supports Multiple Consenter Types
Multimedia and Multilingual (26) Consent Options
Out of the Box Integration with RTSM and EDC
Key Differentiators
Mentor eConsent combines robust tools with participant-centric design—streamlining compliance, customization, and oversight across studies.
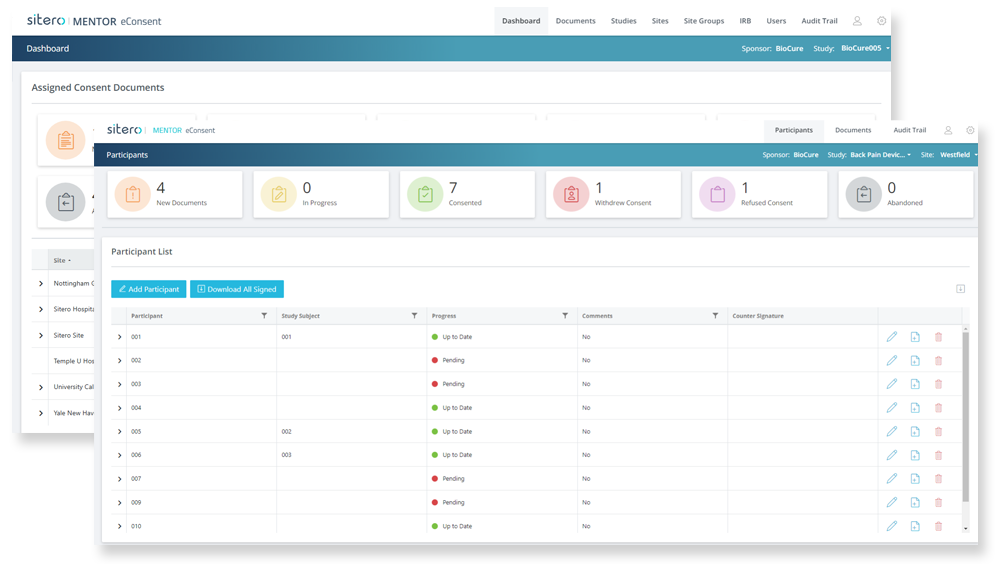
eConsent Accelerated Implementation
Get started quickly with a dedicated implementation team to enable and inform patients today.
Testimonial
“Our team has been impressed with the eConsent platform—it’s intuitive, easy to implement, and has improved participant comprehension. The multimedia tools helped simplify complex study details, leading to faster enrollments and stronger engagement. It’s also streamlined our compliance process and boosted confidence in our consent records.”
Director of Clinical Operations
eConsent Resources
Learn more about digital consent trends and best practices. Explore our latest blog posts and check back soon for new insights.
The future of clinical trials is a unified, interoperable ecosystem. Sitero’s Mentor platform is leading this transformation, ensuring that clinical trials are more efficient, compliant, and streamlined than ever before. Learn more in our latest blog:
We developed this blog as a practical guide for sponsors, CROs, research institutions, and ethics committees (ECs) to successfully adopt eConsent. Learn more: