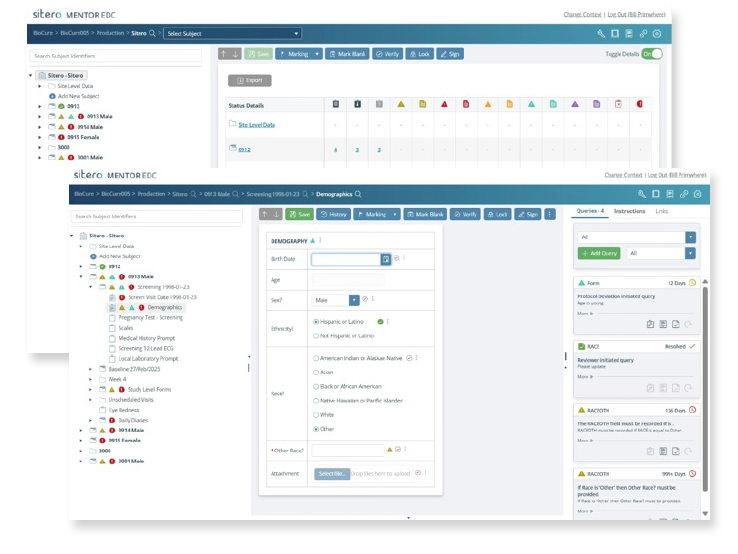
Single Platform. Any Device.
Proven, Powerful, And Scalable Modern Clinical Trial EDC Platform
Sitero’s Mentor EDC platform is a flexible solution that has met the unique requirements of both Sponsors and CROs for over 20 years.
Built to support studies of all sizes and complexities, Mentor EDC delivers speed, accuracy, and real-time access to critical clinical data—on any device, anywhere in the world.
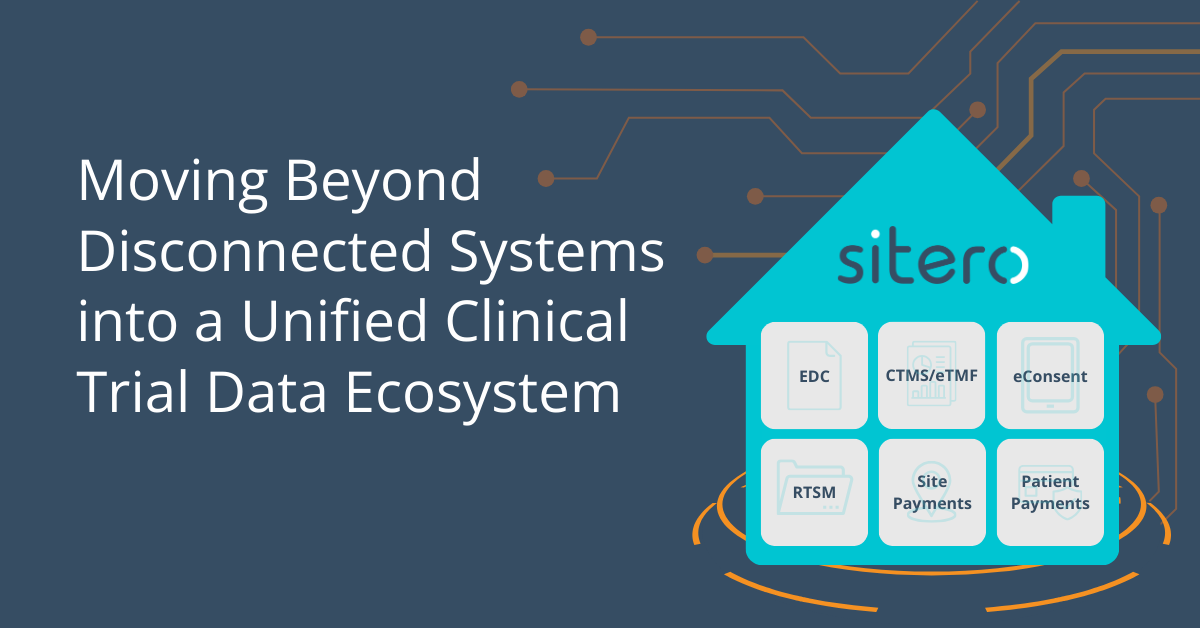
NATIVELY INTEGRATES WITH:
LATEST UPDATES
Major Release Coming May 2025!
We’re continually enhancing Mentor EDC to improve usability, speed, and flexibility. Check back often for the latest platform updates and performance enhancements.

Why Mentor EDC?
Mentor EDC Key Metrics
200+ CUSTOMERS | 3,000+ STUDIES | 13,000+ SITES | 60+ COUNTRIES SERVED
Mentor EDC Features
Trust Sitero to help you find the right approach and scale it appropriately. Mentor EDC offers unmatched flexibility with dynamic forms, eSource readiness, and seamless integration options that meet the needs of today’s trials.
Accelerated Study Builds
Mid-study changes.
No Down-time.
No Migrations.
Advanced Targeted SDV
Data Entry Anywhere, Anytime, by Anyone
Real Time Listings and Summaries
Key Differentiators
Adaptable workflows, built-in capabilities, and real-time data capture—delivering speed, control, and scalability for every study.
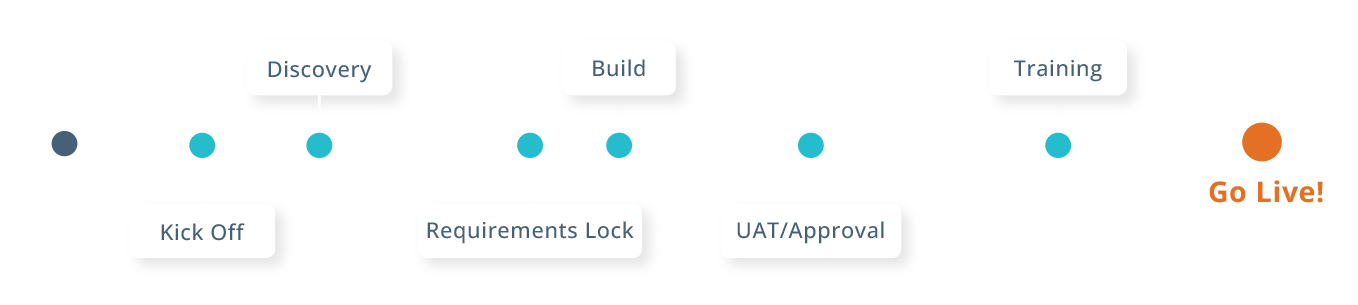
EDC Accelerated Implementation
Our experienced team ensures fast, efficient startup with guided onboarding, best-practice templates, and technology that adapts to your protocol.
Testimonial
“Highly recommend to anyone working at sites that have ever wished a clinical trial system would work better or differently! The Sitero team is incredibly receptive and have made great updates based on feedback in the past, and your feedback would be impactful! I’ve had great experience with the Bioclinica turned Clario turned Sitero EDC system for many years in large part because it is easy for sites, intuitive, and low impact. Making things easy and intuitive for sites is an incredibly critical step for ensuring data quality and accuracy that is often overlooked.”
Former Associate Director, Clinical Data Management
IONIS
EDC Resources
Explore our latest blog posts and product insights. More tools and resources coming soon to support your clinical trial strategies.
Explore how Sitero’s EDC platform helped a leading rare disease sponsor accelerate study builds and improve efficiency through reusable form libraries and trusted collaboration in our latest case study:
The future of clinical trials is a unified, interoperable ecosystem. Sitero’s Mentor platform is leading this transformation, ensuring that clinical trials are more efficient, compliant, and streamlined than ever before. Learn more in our latest blog:
Today’s trials require data entry from anyone, anywhere including patients, sites, home nurses, and remote investigators. Learn why EDC systems must evolve to support decentralized trails in our latest blog: