Moving Beyond Disconnected Systems into a Unified Clinical Trial Data Ecosystem
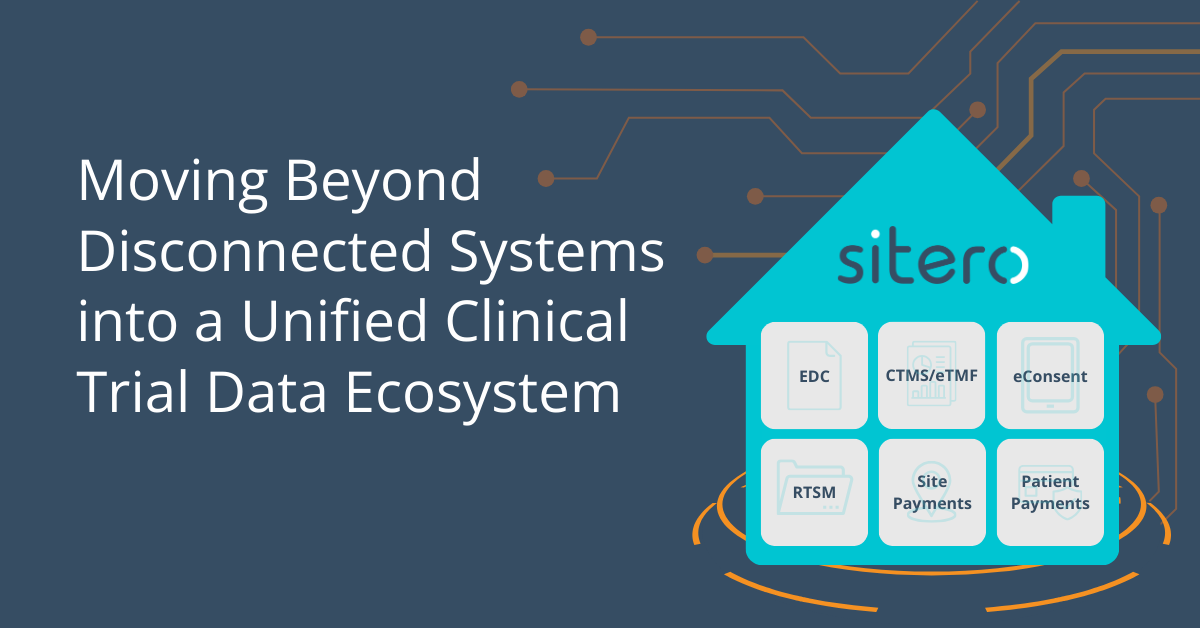
Clinical trials are more complex than ever, yet the technology supporting them is often fragmented, disconnected, and inefficient. Sponsors and CROs rely on multiple standalone systems:
- Electronic Data Capture (EDC)
- Clinical Trial Management System (CTMS)
- Randomization and Trial Supply Management (RTSM)
- eConsent
- Site Payments
- Patient Payments
- Regulatory Compliance
While each system serves a specific function, using them in isolation creates significant challenges across the trial lifecycle. This disjointed approach leads to:
- Data silos that slow decision-making and increase reconciliation efforts
- High operational costs from maintaining multiple systems and integrations
- Redundant workflows that frustrate study teams and sites
- Regulatory challenges due to inconsistent data management and tracking
- A poor site and patient experience from having to navigate multiple platforms
The industry must embrace a unified interconnected clinical trial data ecosystem—one where all study teams, sites, and compliance are seamlessly connected. Achieving this doesn’t mean just integrating systems. It requires true interoperability, where data flows freely between platforms without the need for manual reconciliation.
The Problem: Disconnected Systems Are Slowing Us Down
Most clinical trials today rely on a collection of independent tools that require extensive integrations to work together. While this approach may have worked in the past, it is now a major bottleneck for trial efficiency. Some key factors to consider include:
- Data Silos Create Delays and Errors
- Maintaining Multiple Systems Equals High Operational Costs
- Poor Site and Patient Experience
- Regulatory Compliance Becomes Harder to Manage
Data Silos Create Delays and Errors
EDC, ePRO, CTMS, RTSM, and regulatory systems all collect critical trial data, but they don’t always communicate seamlessly. Site data needs to be manually reconciled across multiple platforms, delaying decision-making and regulatory reporting. A truly interoperable system eliminates silos, ensuring real-time access to complete, high-quality data.
Maintaining Multiple Systems Equals Higher Operational Costs
Sponsors and CROs spend millions integrating and maintaining separate clinical trial systems. Each system requires its own support, training, and configuration, increasing the overall trial burden. A unified ecosystem consolidates costs, reducing the need for expensive integrations and IT maintenance and most importantly streamlines workflows and eases burden on sites and sponsors.
Poor Site and Patient Experience
Sites must switch between different platforms for data entry, patient consent, payments, and compliance tracking. Patients often have to navigate multiple portals for eConsent, ePRO, and study communications, leading to poor engagement, recruitment and enrollment. A single, intuitive interface simplifies interactions for both sites and patients, improving compliance and retention.
Regulatory Compliance Becomes Harder to Manage
Trial data must comply with FDA, EMA, ICH GCP, GDPR, and 21 CFR Part 11 regulations, but disconnected systems make audit trails, reporting, and compliance tracking more difficult. A unified approach ensures that compliance is built into the ecosystem, with a centralized audit trail and automated reporting.
Mentor EDC was purpose-built to meet the challenges of modern clinical research. Unlike legacy platforms that struggle with rigidity and fragmentation, Mentor EDC offers a fully integrated, configurable system that simplifies trial operations and enhances data quality across all trial types.
With Mentor EDC, patients, site staff, and remote providers can all enter data directly into the system with no extra tools or manual handoffs required. The no-code configuration engine allows study teams to design, deploy, and update forms and workflows quickly, supporting the rapid pace of today’s decentralized trials. Built-in ePRO functionality eliminates the need for separate platforms, letting patients report outcomes seamlessly within the same interface.
Mentor also delivers real-time data access and monitoring capabilities, helping sponsors and CROs make informed decisions without delay. Integrated with Mentor CTMS and RTSM systems, Mentor EDC ensures that patient management, randomization, and supply tracking are always in sync. And when it comes to oversight, Mentor EDC supports the full spectrum of monitoring strategies, from traditional on-site reviews to remote and SDV-free models, making it one of the most versatile platforms available for trial execution.
The Solution: A Unified, Interoperable Clinical Trial Data Ecosystem
Instead of relying on fragmented tools, sponsors and CROs should adopt a truly interoperable ecosystem—where all core trial systems work together seamlessly, without the need for constant manual integration.
The Difference Between Interoperability and Integration
Many vendors claim to offer integrated systems, but most are loosely patched tools that require manual data transfers and reconciliations. A truly interoperable ecosystem means that all systems function as one unified platform.
| Integration | Interoperability |
|
|
|
|
|
|
|
|
How Sitero’s Mentor Platform Supports a Unified Ecosystem
With Sitero’s Mentor Platform, sponsors and CROs no longer need to manage disconnected systems—everything is unified, efficient, and built for modern clinical trials. Mentor is designed to support a fully interoperable clinical trial ecosystem by:
✅Providing a seamless connection between EDC, ePRO, CTMS, RTSM, eConsent, and Payments ensuring that all study data flows smoothly between systems.
✅ Eliminates the need for multiple logins and manual data transfers—giving sites, sponsors, and patients a single, intuitive platform to interact and engage.
✅ Ensures real-time data visibility for sponsors and CROs to monitor trial progress instantly without waiting for data reconciliation.
✅ Automates regulatory compliance tracking ensuring that every action is properly documented, reducing audit risks.
Conclusion: The Future of Clinical Trials is a Unified, Interoperable Ecosystem
The future of clinical trials is a unified, interoperable ecosystem. The era of disconnected trial systems is coming to an end. Sponsors and CROs must move beyond patchwork integrations and embrace a truly interoperable ecosystem that connects EDC, ePRO, CTMS, RTSM, eConsent, payments, and regulatory tools in a single, seamless workflow.
Sitero’s Mentor platform is leading this transformation, ensuring that clinical trials are more efficient, compliant, and streamlined than ever before. It’s time to move forward. Contact us to learn more about Sitero’s Mentor technology platform.
Explore how Sitero’s RTSM platform enabled a top 5 pharma company to streamline their randomization and supply management across complex global studies in our latest case study:
Today’s trials require data entry from anyone, anywhere including patients, sites, home nurses, and remote investigators. Learn why EDC systems must evolve to support decentralized trails in our latest blog:




