Simplify Compliance.
Accelerate Your Success.
Smart tools for research oversight
Mentor eCompliance is designed for easy customization and features an intuitive, responsive interface that shortens the learning curve for administrators and users. The platform streamlines reporting, communication, and process management across research, clinical, and higher education institutions.
LATEST UPDATES
Major Releases Coming in June & August 2025!
Check back regularly for the latest improvements and platform news.
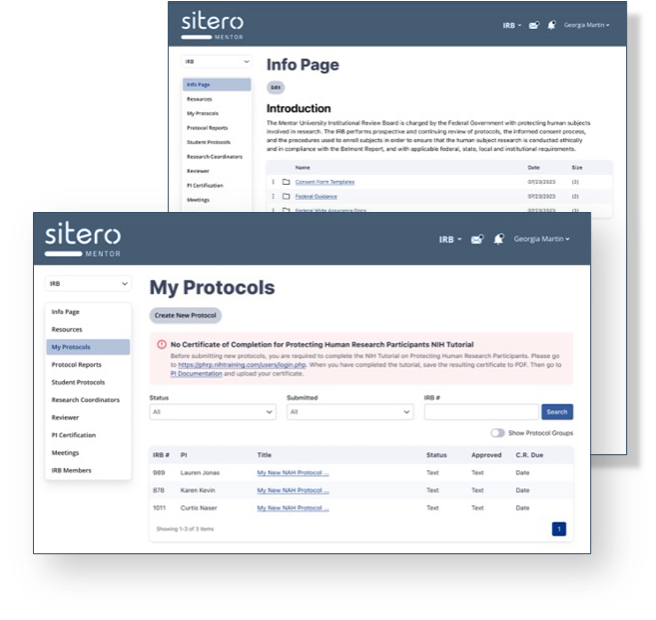
Mentor eCompliance Modules
Mentor streamlines IRB, IBC, IACUC, accreditation record keeping, committee workflows, internship management, course evaluations, and more.
IRB
IACUC
IBC
COI
Education
Grants
Why Mentor eCompliance?
Mentor eCompliance Key Metrics
90+ CLIENTS | 1.5M+ DOCS APPROVED | 90,000+ APPROVED PROTOCOLS
eCompliance Platform Features
Natively Integrated Modules
Cohesive UI/UX Across Modules
Open Architecture (Integrations)
Form Creation / Modification Tool
Standard Form Library
Pre-configured Form Toolkit
Highly Configurable Settings
All Data Capture Fields are Reportable

Hosting & Security
We host Mentor eCompliance on Amazon Web Services, the largest and most secure cloud computing environment in the world.
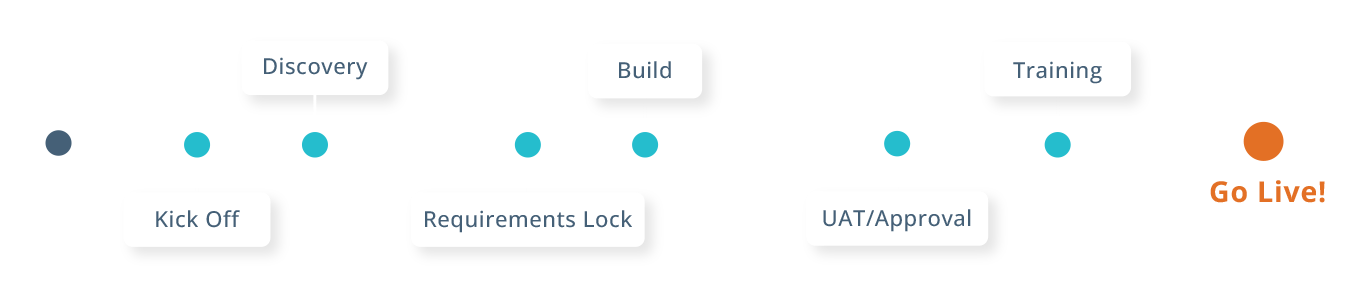
Mentor eCompliance Accelerated Implementation
Get started quickly with a dedicated implementation team to enable and inform patients today.
eCompliance Resources
Explore our latest blog posts and eCompliance resources.
More tools and resources coming soon to support your research administration needs.
Learn about the history and common challenges of human subject research compliance and how organizations can navigate this intricate landscape in our latest blog post:
Preparing for IBC review is complex and time-consuming. Outsourcing IBC services can help streamline the process. Learn more:
Teachers College (TC) partnered with Sitero IRB Services to manage some of their IRB protocol review and administration processes. Learn more in our case study:






