Why EDC Must Evolve to Better Support Decentralized and Hybrid Studies
The way clinical trials are conducted is ever changing. The rise of decentralized and hybrid clinical trials has challenged traditional methods of data collection, yet many electronic data capture (EDC) systems remain rigid. Most legacy EDC platforms were built for on-site data entry, assuming that all trial data would be collected within a clinical setting. But today’s trials require data entry from anyone, anywhere including patients, sites, home nurses, and remote investigators.
As decentralized trials become the new standard, clinical research organizations (CROs), sponsors, and sites must rethink their approach to EDC deployment to ensure trials remain efficient, patient-friendly, and scientifically rigorous. We can achieve this by enabling:
Why Traditional EDC Systems Are No Longer Enough
Most existing EDC systems lack the modernization of integration as well as non-traditional data entry methods which leads to lack of data integrity and frustrations with use.
Today’s patients expect the flexibility to participate from home, and trials increasingly rely on home nurses, telemedicine providers, and remote investigators to collect data. A modern EDC must support all of these roles with a seamless, unified interface that allows for direct patient entry, remote assessments, and traditional site-based transcription—all in one platform. Without intuitive, role-based interfaces, compliance drops and data accuracy suffers.
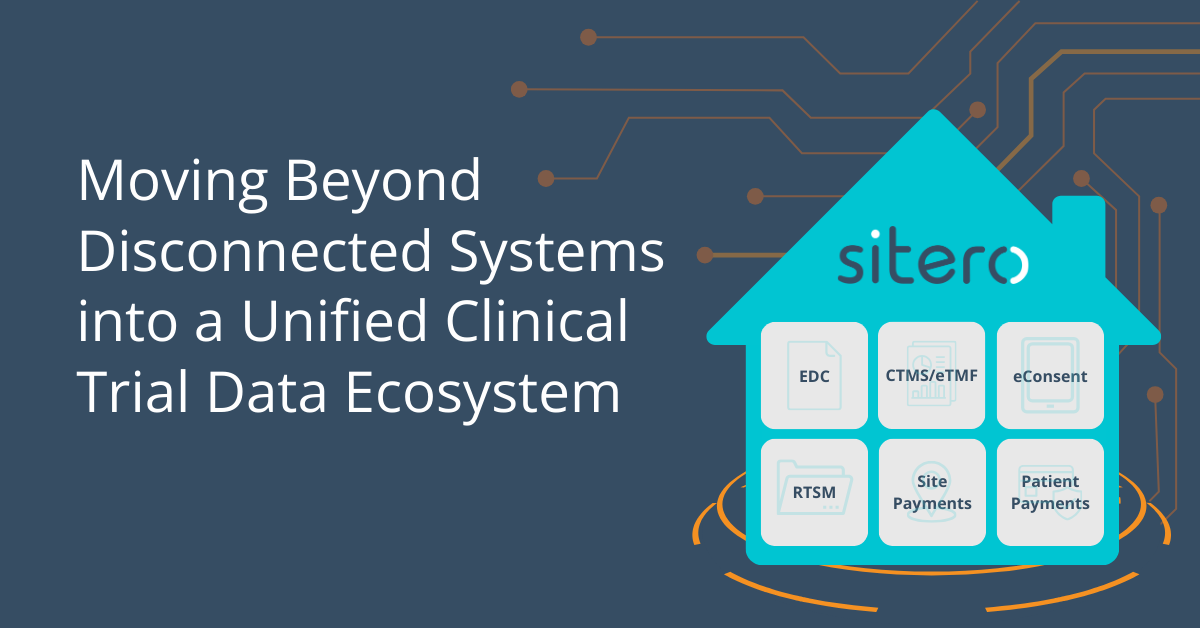
Legacy platforms typically force study teams to juggle multiple disconnected systems, including separate tools for site data entry (EDC), patient-reported outcomes (ePRO), and direct capture (eSource). This fragmentation introduces inefficiencies and unnecessary complexity. Additionally, integration with other systems, like CTMS and RTSM, need to happen in real time, not through batch uploads and manual reconciliation.
Last (and possibly the most important element) is the evolution of multiple monitoring approaches within a single study. While traditional site monitoring still plays a role, modern EDCs should enable remote review and even reduced monitoring through full eSource utilization, where all data is entered directly and verified at the point of capture. A next-generation EDC should unify these capabilities to eliminate redundant tools and workflows, while also giving sponsors the flexibility to choose multiple monitoring approaches best suited to their trial.
The Ideal Modernized EDC
An ideal modernized EDC should do more than just capture data… it should serve as a central, configurable platform that accommodates every participant in the trial ecosystem, from patients and home nurses to site staff and sponsors. First, the system must support both direct data entry and transcription from source.
Patients, remote care providers, and site-based staff should all be able to enter data directly into EDC without relying on external tools. At the same time, study coordinators and investigators should be able to transcribe relevant data when needed, maintaining flexibility in workflows. This type of flexibility also requires a no-code, fully configurable system that allows study teams to adjust forms and workflows in minutes (not days or weeks) ensuring that study designs can be implemented and iterated on with ease.
Additionally, a modernized EDC must include built-in ePRO functionality. Too often, patient-reported outcomes require a separate system and vendor integration, creating unnecessary complexity. With native ePRO support, patients can provide data directly within the EDC, streamlining the experience for both participants and study teams.
Real-time data capture and monitoring tools are also critical. Sponsors and CROs need immediate visibility into study progress, with alerts and dashboards that surface potential issues early. And finally, seamless integration with CTMS and RTSM is essential to ensure smooth coordination across study operations, patient enrollment, and drug supply. To support diverse trial models, the system should also enable flexible monitoring approaches. Whether using traditional site visits, remote review, or reduced SDV workflows enabled by eSource, the EDC must adapt to the monitoring strategy that best fits the study design.
How Mentor EDC Sets the Standard for Modern Platforms
Mentor EDC was purpose-built to meet the challenges of modern clinical research. Unlike legacy platforms that struggle with rigidity and fragmentation, Mentor EDC offers a fully integrated, configurable system that simplifies trial operations and enhances data quality across all trial types.
With Mentor EDC, patients, site staff, and remote providers can all enter data directly into the system with no extra tools or manual handoffs required. The no-code configuration engine allows study teams to design, deploy, and update forms and workflows quickly, supporting the rapid pace of today’s decentralized trials. Built-in ePRO functionality eliminates the need for separate platforms, letting patients report outcomes seamlessly within the same interface.
Mentor also delivers real-time data access and monitoring capabilities, helping sponsors and CROs make informed decisions without delay. Integrated with Mentor CTMS and RTSM systems, Mentor EDC ensures that patient management, randomization, and supply tracking are always in sync. And when it comes to oversight, Mentor EDC supports the full spectrum of monitoring strategies, from traditional on-site reviews to remote and SDV-free models, making it one of the most versatile platforms available for trial execution.
Conclusion: The Future of EDC
As decentralized and hybrid trials redefine how clinical research is conducted, legacy EDC systems are falling behind. Built for site-based workflows, they often lack the flexibility, integration, and configurability today’s studies require. Moving forward, EDC platforms must enable real-time access, direct data entry from any source, and seamless interoperability across study systems.
Sitero’s Mentor EDC was designed to meet these needs by streamlining operations and supporting modern trial models without added complexity. With features that simplify study design and execution, Mentor empowers sponsors and CROs to keep pace with the future of clinical research:
- No-code configurability
- Native ePRO within the EDC
- Real-time data access and monitoring
- Seamless integration with CTMS and RTSM
Mentor EDC sets the standard, eliminating inefficiencies and ensuring that clinical research remains patient-centric, efficient, and future-proof.
The future of clinical trials is here. Is your EDC system ready? Contact us today to learn more.
Explore how Sitero’s EDC platform helped a leading rare disease sponsor accelerate study builds and improve efficiency through reusable form libraries and trusted collaboration in our latest case study:
The future of clinical trials is a unified, interoperable ecosystem. Sitero’s Mentor platform is leading this transformation, ensuring that clinical trials are more efficient, compliant, and streamlined than ever before. Learn more in our latest blog: